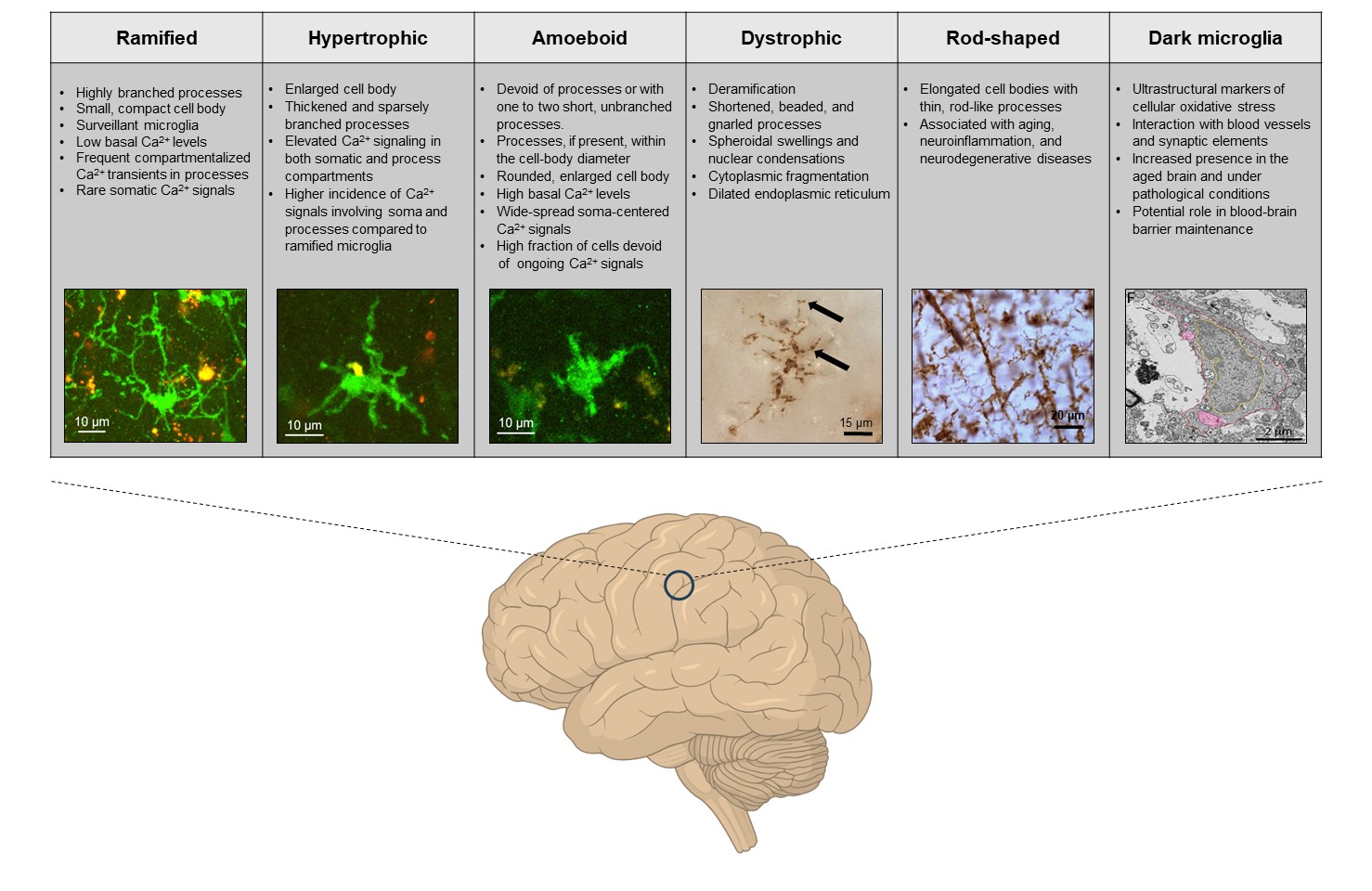
 Microglia, the resident immune cells of the central nervous system, exhibit a wide array of functional states, even in their so-called “homeostatic” condition, when they are not actively responding to overt pathological stimuli. These functional states can be visualized using a combination of multi-omics techniques (e.g., gene and protein expression, posttranslational modifications, mRNA profiling, and metabolomics), and, in the case of homeostatic microglia, are largely defined by the global (e.g., genetic variations, organism’s age, sex, circadian rhythms, and gut microbiota) as well as local (specific area of the brain, immediate microglial surrounding, neuron-glia interactions and synaptic density/activity) signals (Paolicelli et al., 2022).
Microglia, the resident immune cells of the central nervous system, exhibit a wide array of functional states, even in their so-called “homeostatic” condition, when they are not actively responding to overt pathological stimuli. These functional states can be visualized using a combination of multi-omics techniques (e.g., gene and protein expression, posttranslational modifications, mRNA profiling, and metabolomics), and, in the case of homeostatic microglia, are largely defined by the global (e.g., genetic variations, organism’s age, sex, circadian rhythms, and gut microbiota) as well as local (specific area of the brain, immediate microglial surrounding, neuron-glia interactions and synaptic density/activity) signals (Paolicelli et al., 2022).